We investigate the ways in which the central nervous system controls movement through study of the motor unit: the key structure that transforms neural commands that converge upon it in the spinal cord into motor output through the generation of muscle force. Analysis of motor unit population discharge allows the study of voluntary motor commands at the end-stage of central nervous system processing. In this way, it captures contributions to motor processing not only from the brain, but also from the brainstem, spinal cord, and a-motoneuron—structures that are implicated after neurological injury but are often overlooked in human neurorehabilitation.
We measure motor unit discharge non-invasively in humans using high-density surface EMG recordings (in up to six muscles simultaneously) coupled with an automatic decomposition algorithm (Negro et al., 2016). By performing various time- and frequency-domain analyses on the decomposed motor unit spike trains, we can “decode” information about the synaptic inputs that the motoneurons received and thereby characterize the voluntary motor command at the end-stage of central nervous system processing. This approach also allows us to record from many motor units simultaneously (rather than only a few), which is a major advantage because we can 1) extract much more detailed information about the characteristics of synaptic input to the motoneuron pool and 2) obtain robust population distributions from individuals in addition to group analyses. The recent development of this advanced technology enables us to address scientific and clinical research questions that were previously infeasible.
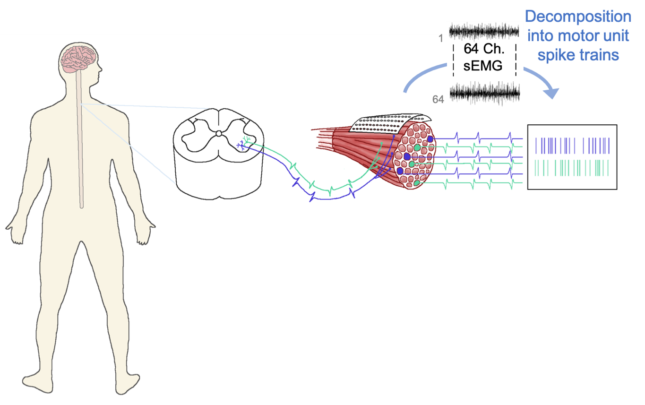
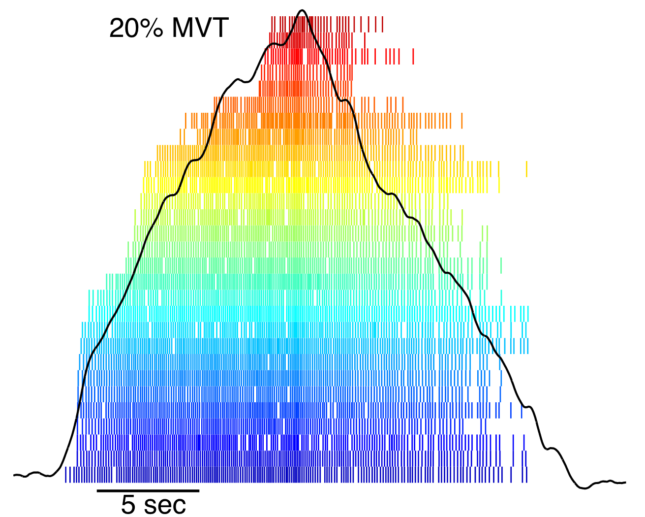

One line of research of our lab is to characterize pathological motoneuron discharge following injury to the central nervous system (e.g., multiple sclerosis, stroke) and to determine the ways in which it is associated with specific clinical motor deficits. These findings will identify which pathophysiological processes may be promising therapeutic targets and ascertain the therapeutic modalities that are most likely to be beneficial. Our short-term goals are to quantify and characterize the ways in which neural drive to muscle is altered these conditions, and to evaluate the ways in which pathologic motor unit discharge is associated with typical motor deficits. The long-term goals of our work are to increase patient function by (1) informing the development of targeted, personalized interventions, (2) by elucidating physiologic changes associated with current interventions, and (3) by providing sensitive disease monitoring tools.
Another line of research in our lab is to study differences in neural control of proximal and distal muscles, which have different biomechanical functions and neural innervation patterns. This work provides foundational knowledge about function of the intact nervous system that is useful when seeking to understand why motor deficits following neurological injury often differ among proximal and distal muscles.
We also collaborate with labs conducting pre-clinical research using animal paradigms (cat, rat, rabbit), which facilitate our ability to validate and interpret our findings from human experiments because the animal models provide a ground-truth measure of central nervous system output by permitting direct, invasive measurements from nervous system.
For more information, please see Dr. Laura McPherson’s laboratory website: https://sites.wustl.edu/snrlab
Faculty Investigator
Laura McPherson, PT, DPT, PhD [Profile ![]() ]
]
Lab Members

Daniel Free, PhD
Staff Scientist

Skyler Simon, BS
Senior Research Technician

Krystal Panagiotaros, BS, SPT
DPT Student Research Assistant

Abbey Painter, BS
Research Assistant I

Rin Limi
Undergraduate Research Assistant
Current Research Studies
Neural mechanisms of motor heterogeneity in multiple sclerosis (McPherson: PI) Washington University ICTS KL2 Career Development Award
Subclinical pathophysiology of motor processing in people with multiple sclerosis (McPherson: PI) Department of Defense CDMRP Multiple Sclerosis Research Program Exploration Hypothesis-Generation Award
Supercomputer-based Models of Motoneurons for Estimating their Synaptic Inputs in Humans (Heckman (PI), McPherson et al. (co-I))NIH/NINDS R01